This article describes the new Leapfrog quality metrics and their methodology, focusing on postoperative sepsis identification and the potential impact of dashboard performance tracking moving forward. Critical care professionals routinely encounter patients with sepsis and play an integral role in the formulation and implementation of management plans for postoperative sepsis, making them key participants in this effort.
Leapfrog Hospital Safety Grade is a national rating system of U.S. hospitals developed in the 1990s by a healthcare quality improvement coalition. Hospitals participating in an inpatient prospective payment system of the Centers for Medicare and Medicaid Services (CMS) are surveyed on more than 30 performance measures and graded on a scale of A to F to reflect the hospitals’ infrastructure and performance on patient safety measures,
1 including healthcare-associated infections (HAIs). In 2009, the U.S. Department of Health and Human Services (HHS) released a national action plan focused on preventing HAIs in acute care hospitals, including central line-associated bloodstream infection (CLABSI), catheter-associated urinary tract infection (CAUTI), surgical site infection (SSI), methicillin-resistant
Staphylococcus aureus (MRSA) bloodstream infections, and
Clostridiodes difficile infection (CDI). In 2011 CMS mandated reporting HAI data through the National Healthcare Safety Network (NHSN).
2 Despite these efforts, HAIs continue to affect 4% of all hospitalized patients with device-associated infections, together accounting for 25.6% of cases, and SSI alone accounting for 21.6% of cases.
3 Leapfrog uses reduction in HAIs as a quality metric in its latest web-based fall 2021 dashboard.
4
The updated
Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021, published by the Society of Critical Care Medicine (SCCM) and the European Society Intensive Care Medicine (ESICM), included recommendations on source control in sepsis.
5 Given the high prevalence of SSIs, the implementation of this recommendation, among others, is likely to influence Leapfrog Hospital Safety Grade by directly affecting the rates of postoperative sepsis, which are now part of a composite patient safety indicator (PSI).
In this article, we describe the new Leapfrog quality metrics and their methodology, focusing on postoperative sepsis identification and the potential impact of dashboard performance tracking moving forward. Critical care professionals routinely encounter patients with sepsis and play an integral role in the formulation and implementation of management plans for postoperative sepsis, making them key participants in this effort.
Leapfrog Methodology as the Performance Measure for U.S. Hospitals
Guided by an expert panel, the Leapfrog methodology focuses on patient safety, defined as “freedom from harm.” It uses an open-access online dashboard
4 that helps visualize safety measures in individual hospitals and helps comparison across hospitals. The concept of clinical dashboards as a tool to demonstrate current progress toward specific goals has a long history in the business arena; more recently clinical dashboards have been adopted within healthcare to improve interconnectivity and patient care knowledge among clinicians, healthcare administrators, information technology specialists, quality officers, and nursing and other ancillary department leaders. The dashboards also support transparency, decision-making, and dissemination of information, aiding the formulation of new hospital polices.
Leapfrog places equal weight on 1) process and structural measures and 2) outcome measures. Process measures include hospital staff responsiveness and adherence to the CMS/Joint Commission Surgical Care Improvement Project (SCIP). Structural measures include computerized physician order entry for medications. Outcome measures include several PSIs, of which HAIs form the largest percentage.
6 In fall of 2021, the composite PSI-90, which combines 10 PSIs and accounts for 15% of the overall grade, was introduced (
Figure 1).
Figure 1. Hospital Safety Grade Outcome Measures Estimated Standard Measures Weights: Fall 2021.
 |
| PSI-90 includes rates of pressure ulcer (PSI-3), iatrogenic pneumothorax (PSI-6), in-hospital fall with hip fracture (PSI-8), perioperative hemorrhage and hematoma (PSI-9), postoperative acute kidney injury (PSI-10), postoperative respiratory failure (PSI-11), perioperative pulmonary embolism or deep venous thrombosis (PSI-12), postoperative sepsis (PSI-13), postoperative wound dehiscence (PSI-14), and unrecognized abdominopelvic accidental puncture/laceration (PSI-15). |
Continuous, concurrent tracking of these measures with tools such as dashboards provides the foundation for transformative discussion that can guide innovative process management7 and tracking of intervention effects. When developing a dashboard, facilities should examine the complete report from their most recent Leapfrog evaluation and identify underperforming measures. Data sources for each of the measures should be clearly identified so that the concurrent dashboard can be populated with updated information at regular intervals, typically monthly. Most of the process and structural measures can be manually extracted from the electronic medical record, staffing systems, facility hand hygiene observation process, or the Leapfrog survey. Outcome measures can typically be obtained by manual chart extraction or with a claims-based quality management system such as Premier or Vizient using CMS and NHSN reports as the data source.
Ideally, every facility would create a custom dashboard that would allow for accurate visualization of trends and practice patterns. On one hand, this would increase accountability for individual and group practices; on the other hand, it would predict overall facility Leapfrog grading. For key metrics or areas of focus, it may be helpful to include front line drivers (FLD),8 which are actions that staff can take to affect specific outcome measures. Figure 2 depicts a hospital safety grade dashboard wherein FLD for PSI-13 (postoperative sepsis) were identified as handwashing and CMS Sep-1 bundle compliance in the surgical ICU. In the example, improvement in FLD corresponds with improvement in PSI-13 and PSI-90 scores. Once dashboards have been created and the data population has been delineated, it is essential to identify a plan for dissemination to all stakeholders either electronically or at a hospital committee with strong multidisciplinary leadership attendance.
Figure 2. Example of Hospital Safety Grade Dashboard with Frontline Drivers
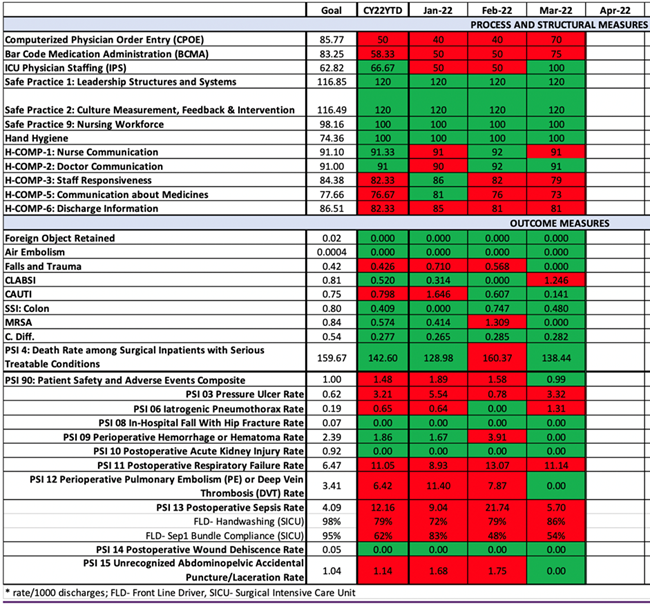
Facility leaders should use the dashboards to help direct decision-making regarding targeted resource allocation;10 implementation of measure-specific action plans, including pertinent clinical practice guidelines; and real-time tracking of the effects of such interventions, allowing for immediate modifications and long-term planning. For example, an increase in CLABSI at a facility would be reflected in the dashboard and would trigger an analysis of the cases, with possible identification of common risk factors (e.g., catheter insertion by the same clinician or team). Thus, knowledge and skill assessment of the clinician or team could occur, regarding infection prevention measures, provision of education, and tracking their future practices as reflected by their future rates of CLABSI. The real-time analysis would allow for early identification and corrective measures, which could prevent overall performance from being significantly affected, resulting in maintenance and improvement of patient safety scores.
Source Control for Postoperative Sepsis in Light of the Surviving Sepsis Campaign
The Agency for Healthcare Research and Quality (AHRQ) defines postoperative sepsis rate (PSI-13) as the number of cases of postoperative sepsis as a secondary diagnosis per 1000 elective surgical discharges for patients aged 18 years and older. This encompasses sepsis from all causes, including shock, and of any severity in the postoperative period. The presence of sepsis on admission as a principal or secondary diagnosis is a criterion for case exclusion from this rate calculation, as is postoperative infection without evidence of sepsis.
In the United States, around 1.2% of all elective surgery patients develop sepsis in the postoperative period. Hospital length of stay (18 days vs. 6 days, P < 0.0001) and hospital mortality (25.88% vs. 0.81%, P < 0.0001) for postoperative sepsis are substantially higher than in those without sepsis.11 This trend toward higher mortality continues for up to 1 year after hospital discharge.12 Abdominal procedures involving the gastrointestinal tract other than the colon (esophageal, gastric, small bowel, pancreatic) are associated with the highest mean risk-adjusted incidence rates of more than 2.75%. Despite lower incidence, sepsis after noncardiac thoracic procedures was associated with the highest risk-adjusted hospital mortality, approaching 46%. Moreover, there is a substantial monetary cost. In the 2002-2006 cohort from the AHRQ-sponsored nationwide inpatient sample, the median total hospital stay cost was substantially more for those with postoperative sepsis ($57,032 vs. $17,229, P < 0.0001).11 These numbers do not consider the morbidity associated with such severe illness and the cost of ongoing out-of-hospital care for subsequent infections and complications. This imposes a significant and potentially reducible burden on healthcare systems. A 10% reduction in postoperative sepsis from general surgical procedures alone could reduce healthcare costs by $421 million.13
Early (1-3 days) postoperative infections may be community-acquired preoperatively, but late (4-30 days) postoperative infections are largely site-specific SSIs and may be superficial, deep incisional, or deep space/organ related. HAIs can occur at any time in the postoperative course and are more likely to be associated with multidrug-resistant (MDR) organisms and postoperative critical illness when indwelling catheters and devices are used.
The Surviving Sepsis Campaign adult guidelines5 emphasize early and definitive control of the source of infection, defined as “identification of a specific anatomical diagnosis contributing to infection and subsequent intervention to remove it,” as a best practice recommendation. Included in this recommendation is definitive removal of a source of ongoing microbial contamination such as infected skin and soft tissue; infected indwelling catheters or intracardiac devices; infected fluid collections such as intra-abdominal abscess, empyema, or superinfected seroma or hematoma; and, in dire circumstances, amputation or removal of vital organs such as in traumatic or spontaneous necrotizing infections. Also included in the recommendation is limiting the introduction of otherwise site-specific normal flora into a relative sterile space such as the peritoneum in the case of bowel perforation by correction of anatomical derangements. Such efforts often also establish the microbial diagnosis and help guide optimal antimicrobial strategies.
Because of the varied nature of such infection sources, definitive data about the effect of source control measures on clinical outcomes is primitive at best. The least invasive and most effective strategy is recommended and, the earlier the intervention, the better. For critically ill patients, open surgical drainage may be precluded by hemodynamic instability, favoring percutaneous or endoscopic approaches or suboptimal drainage procedures referred to as damage control. For necrotizing fasciitis, earlier source control, within 6 to 12 hours, is associated with substantially reduced mortality.14 Such a time cutoff has not been identified in other sites or infection types, although increasing data suggest that, for severe sepsis or septic shock, delays are associated with higher mortality,15 especially in those with an intra-abdominal source.16-19 However, adequate source control is associated with improved clinical outcomes and survival, even if performed later in the course of illness.15,17,20,21 In the case of MDR organisms, adequate source control has been independently associated with improved clinical outcomes.22
We strongly advocate for the three-pronged approach of appropriate antimicrobial therapy, adequate source control, and supportive care for the treatment of all patients with sepsis or septic shock. The key, however, to decreasing the rate of postoperative sepsis is in the adequate implementation of guideline-directed preventative measures that have been proven to decrease HAIs,23-27 such as strict adherence to hand hygiene measures, appropriate indication for devices and removal when no longer necessary, appropriate insertion and maintenance techniques, and strong antibiotic stewardship interventions.
Conclusion
Data are scarce on the impact of implementing Leapfrog Hospital Safety Grade on overall clinical outcomes. However, using dashboards to track specific parameters allows for objective assessment and better resource utilization and monitoring progress, ultimately improving patient care and hospital performance.
References
- Austin JM, D’Andrea G, Birkmeyer JD, et al. Safety in numbers: the development of Leapfrog’s composite patient safety score for U.S. hospitals. J Patient Saf. 2014 Mar;10(1):64-71. doi:10.1097/PTS.0B013E3182952644
- Yokoe DS, Anderson DJ, Berenholtz SM, et al. A compendium of strategies to prevent healthcare-associated infections in acute care hospitals: 2014 updates. Infect Control Hosp Epidemiol. 2014 Sep;35 Suppl 2:S21-S31.
- Magill SS, Edwards JR, Bamberg W, et al; Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014 Mar 27;370(13):1198-1208. doi:10.1056/NEJMoa1306801
- Leapfrog Group. Leapfrog Hospital Safety Grade. Scoring Methodology Fall 2021 Safety Grade. Last updated October 25, 2021. Accessed March 4, 2022. https://www.hospitalsafetygrade.org/media/file/Safety-Grade-Methodology-Fall-2021.pdf
- Evans L, Rhodes A, Alhazzani W, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021 Nov 1;49(11):e1063-e1143. doi:10.1097/CCM.0000000000005337
- Austin JM, Jha AK, Romano PS, et al. National hospital ratings systems share few common scores and may generate confusion instead of clarity. Health Aff (Milwood). 2015 Mar;34(3):423-430. doi:10.1377/hlthaff.2014.0201
- Porter ME. What is value in health care? N Engl J Med. 2010 Dec 23;363(26):2477-2481.
- McKinney J. Accelerate hospital process improvements with front line drivers: Make real-time improvements with real-time data. Tier1 Performance. Accessed January 16, 2022. https://tier1performance.com/accelerate-hospital-improvements-with-front-line-drivers/. 2021.
- Ogrinc GO, Headrick LA, Barton AJ, Dolansky MA, Madigosky WS, Miltner RS, eds. Fundamentals of Health Care Improvement: A Guide to Improving Your Patients’ Care. 3rd ed. Joint Commission Resources; 2018.
- NAHQ, Pelletier LR, Beaudin CL, eds. HQ Solutions: Resource for the Healthcare Quality Professional. Lippincott Williams and Wilkins; 2017.
- Vogel TR, Dombrovskiy VY, Carson JL, Graham AM, Lowry SF. Postoperative sepsis in the United States. Ann Surg. 2010 Dec;252(6):1065-1071. doi:10.1097/SLA.0b013e3181dcf36e
- Ou L, Chen J, Hillman K, et al. The impact of post-operative sepsis on mortality after hospital discharge among elective surgical patients: a population-based cohort study. Crit Care. 2017 Feb 20;21(1):34. doi:10.1186/s13054-016-1596-7
- Vaughan-Sarrazin MS, Bayman MA, Cullen JJ Costs of postoperative sepsis: the business case for quality improvement to reduce postoperative sepsis in Veterans Affairs hospitals. Arch Surg. 2011 Aug;146(8):944-951. doi:10.1001/archsurg.2011.78
- Chao WN, Tsai CF, Chang HR, et al. Impact of timing of surgery on outcome of Vibrio vulnificus-related necrotizing fasciitis. Am J Surg. 2013 Jul;206(1):32-39. doi:10.1016/j.amjsurg.2012.08.008
- Bloos F, Thomas-Rüddel D, Rüddel H, et al; MEDUSA Study Group. Impact of compliance with infection management guidelines on outcome in patients with severe sepsis: a prospective observational multi-center study. Crit Care. 2014 Mar 3;18(2):R42. doi:10.1186/cc13755
- Boyd-Carson H, Doleman B, Cromwell D, et al; National Emergency Laparotomy Audit Collaboration. Delay in source control in perforated peptic ulcer leads to 6% increased risk of death per hour: a nationwide cohort study. World J Surg. 2020 Mar;44(3):869-875. doi:10.1007/s00268-019-05254-x
- Azuhata T, Kinoshita K, Kawano D, et al. Time from admission to initiation of surgery for source control is a critical determinant of survival in patients with gastrointestinal perforation with associated septic shock. Crit Care. 2014 May 2;18(3):R87. doi:10.1186/cc13854
- Buck DL, Vester-Andersen M, Møller MH; Danish Clinical Register of Emergency Surgery. Surgical delay is a critical determinant of survival in perforated peptic ulcer. Br J Surg. 2013 Jul;100(8):1045-1049. doi:10.1002/bjs.9175
- Karvellas CJ, Abraldes JG, Zepeda-Gomez S, et al; Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group. The impact of delayed biliary decompression and anti-microbial therapy in 260 patients with cholangitis-associated septic shock. Aliment Pharmacol Ther. 2016 Oct;44(7):755-766. doi:10.1111/apt.13764
- Kim H, Chung SP, Choi SH, et al; Korean Shock Society (KoSS) Investigators. Impact of timing to source control in patients with septic shock: a prospective multi-center observational study. J Crit Care. 2019 Oct;53:176-182. doi:10.1016/j.jcrc.2019.06.012
- Martínez ML, Ferrer R, Torrents E, et al. Impact of source control in patients with severe sepsis and septic shock. Crit Care Med. 2017 Jan;45(1):11-19. doi:10.1097/CCM.0000000000002011
- Falcone M, Russo A, Iacovelli A, et al. Predictors of outcome in ICU patients with septic shock caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Clin Microbiol Infect. 2016 May;22(5):444-450. doi:10.1016/j.cmi.2016.01.016
- Anderson DJ, Podgorny K, Berríos-Torres SI, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014 Jun;35(6):605-627. doi:10.1086/676022
- Lo E, Nicolle LE, Coffin SE, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(5):464-479. doi:10.1086/675718
- Yokoe DS, Anderson DJ, Berenholtz SM, et al; Society for Healthcare Epidemiology of America (SHEA). A compendium of strategies to prevent healthcare-associated infections in acute care hospitals: 2014 updates. Infect Control Hosp Epidemiol. 2014 Aug;35(8):967-977. doi:10.1086/677216
- Dubberke ER, Carling P, Carrico R, et al. Strategies to prevent Clostridium difficile infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014 Jun;35(6):628-645. doi:10.1086/676023
- Klompas M, Branson R, Eichenwald EC, et al; Society for Healthcare Epidemiology of America (SHEA). Strategies to prevent ventilator-associated pneumonia in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014 Aug;35(8):915-936. doi:10.1086/677144






