The Society of Critical Care Medicine (SCCM) invites collaboration with industry partners seeking to support and advance research in critical care.
Through its research arm, Discovery, the Critical Care Research Network, SCCM offers a trusted infrastructure for administering investigator-led research grants. SCCM, a 501(c)(3) charitable organization, welcomes interest forms focused on a broad range of clinical and translational research topics. Industry grant sponsors may include life sciences companies, medtech and digital health firms, and other mission-aligned organizations.
This opportunity is ideal for organizations looking to:
To learn more, please contact Brian Fitzgerald or complete the interest form.
Industry-Funded Research Grants: Aimed at supporting novel therapies, clinical studies, and translational research projects. Research may address clinical topics such as sepsis, acute respiratory distress syndrome, trauma, and organ dysfunction.
Industry-Funded Innovation Grants: Aimed at fostering the development and implementation of innovative technologies or devices to improve patient care in critical care settings. Examples include studies on the development of medical devices, software applications, or telemedicine solutions.
Other Industry-Funded Grants: Interest is broad. Other grant types may include those aimed at enhancing the quality, safety, and efficiency of critical care delivery. Examples include studies focused on implementing evidence-based practices, optimizing care processes, or reducing healthcare-associated infections.
Industry grant sponsors have the opportunity to align their brand and values with SCCM while supporting research that propels critical care forward.
The process of sponsoring a grant starts by completing the interest form. Be prepared with the following information:
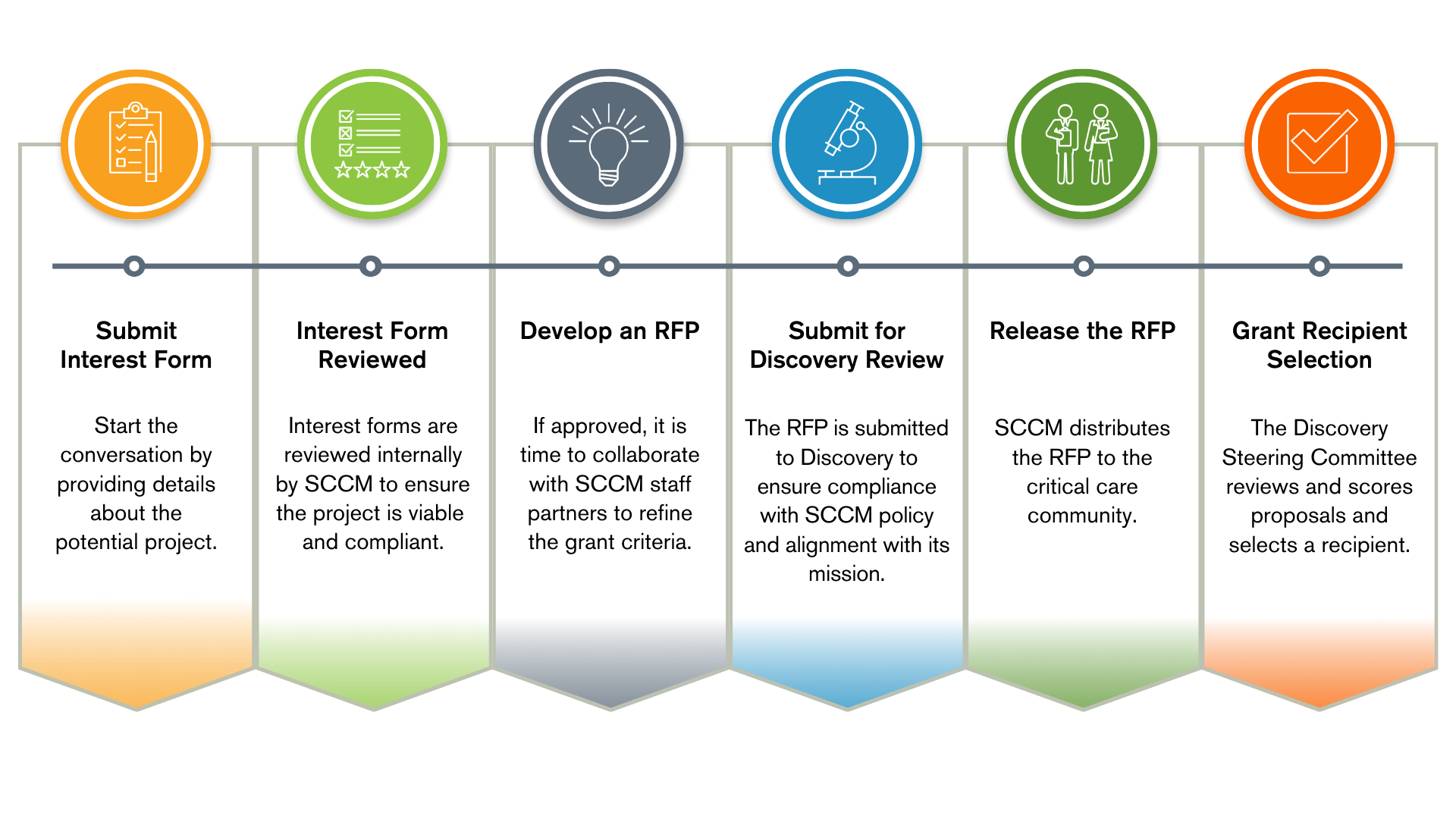
Once an interest form is submitted and approved, SCCM will work with the industry grant sponsor to refine the scope of the project (eligibility criteria, application requirements and deadlines) and develop a Request for Proposals (RFP).
The Discovery Steering Committee reviews RFPs to ensure they align with SCCM policies and the rules and considerations for industry-sponsored grants.
Industry grant sponsors must agree to the following: